Hello,
I am Ashwini Rajasekaran
A Research Scientist pioneering in Molecular Biology, where curiosity meets innovation. With a passion for unraveling the intricacies of life at a molecular level, harnessing cutting-edge technologies to advance our understanding of biological systems, my goal is to work towards designing novel therapeutic modules of complex disorders.

Resilience in Every Step
Research Experience
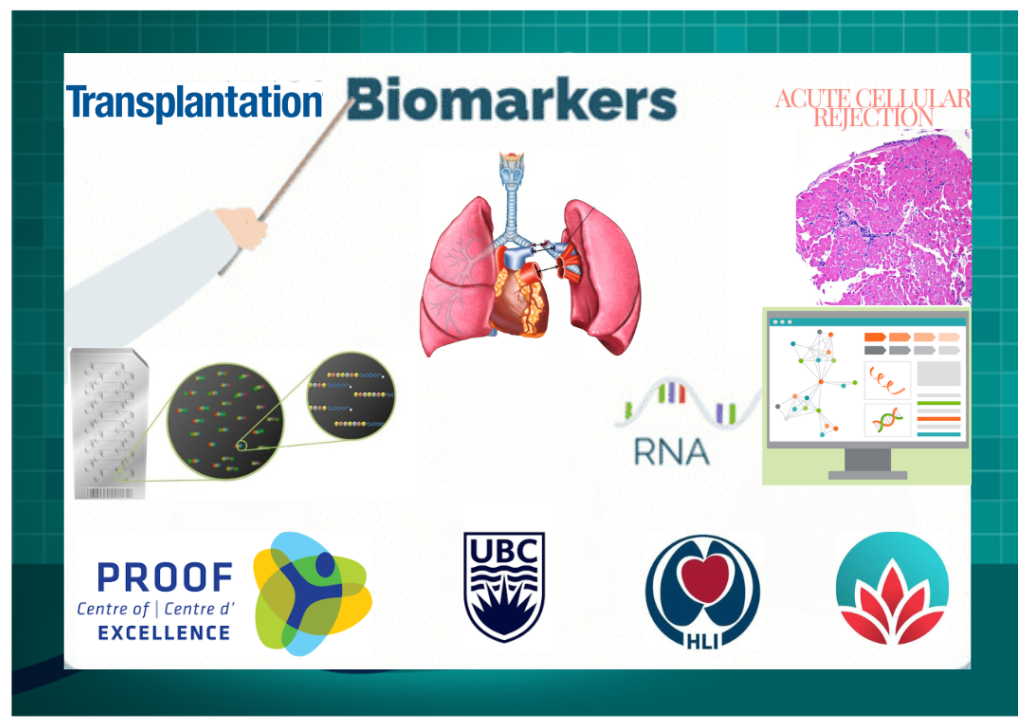
Tech Scientist (Staff)
Centre for Heart and Lung Institute, Core-1 laboratory, UBC, Vancouver, Canada
Feb 2024 - Present
My research involves working in a multidisciplinary team comprising of biomedical researchers, clinicians, computational biologists, and data scientists. The teams’ overarching goal is to develop biomarker panels for accurate diagnosis of a range of medical complications and conditions such as organ transplant rejections, interstitial lung diseases, allergic rhinitis and occupational asthma. I independently lead operations for a laboratory at the Core-1 molecular phenotyping facility in the research wing of St.Paul’s hospital and NetCAD, the research wing of Canadian Blood services located at University of British Columbia, Vancouver. I orient the research team with experimental designs, execution, data generation and analysis, grant preparations, budgeting and laboratory management.

Industrial Postdoctoral Fellow
Institute of Pharmacology and Physiology, Université de Sherbrooke, Québec, Canada & deutraMed, Ontario, Canada
MITACS industrial postdoctoral fellow:
MITACS awarded me an industrial postdoctoral fellowship, to research on RNA therapeutics for neuro-inflammatory diseases, in collaboration with an industrial partner, DeutraMed and academic partner, Université de Sherbrooke. I led the project towards develop D-Lock TM Stabilization Technology. This is deuterium-based mRNA stabilization technology aimed at developing a stable RNA-based vaccine. This patented technology offers a promising solution to the significant cost and challenge of cold-chain logistics. Upon successfully developing a stable in-vitro synthesized mRNA, I explored the therapeutic role of an innate immune component of pattern recognition receptor called NLRX1 in multiple sclerosis. I tested it in experimental mouse model of MS using lipid nanoparticles. I explored the immunogenicity of the stable mRNA in human and mouse cell lines.

Scientist, Translational Research in Occupational Asthma
PROOF Centre of Excellence, St. Paul’s Hospital, Vancouver, Canada
Postdoctoral Scientist, Translational Research in Occupational Asthma:
Biotalent Canada awarded me a grant to research on blood-based biomarker discovery, in collaboration between PROOF Centre for excellence and Centre for Heart & Lung Innovation. I executed a discovery study to develop biomarker for occupational asthma subtypes and validated using machine learning analytic tools. During this tenure, I conceptualized genetic analysis to identify SNPs associated with asthmatic responses. My work during this period led to discovery of cholinergic synapse pathway gene polymorphisms association with allergen-induced late asthmatic responses, which was then published in European respiratory journal.

Research Associate and Team Lead
KPU, Surrey and University of Toronto, Canada
I worked with the Canadian Longitudinal Study on Aging (www.CLSA.ca). This work was with a national team of collaborators from the University of Toronto, University of Victoria, Ryerson University, University of Ottawa, and MacEwan University to examine various health determinants and their relationships with physical, mental, and cognitive health outcomes. Another project I worked on, investigated practitioner-led peer supported personalized nutrition approaches that are based on nutrigenomics provided in an interactive online virtual environment. Part of the study included collecting eye tracking data to better understand how people interact with health information presented in online social environments. The goal of the project was to discover whether these approaches can foster positive and sustained healthbehaviour changes in emerging adults (18 to 25 years).
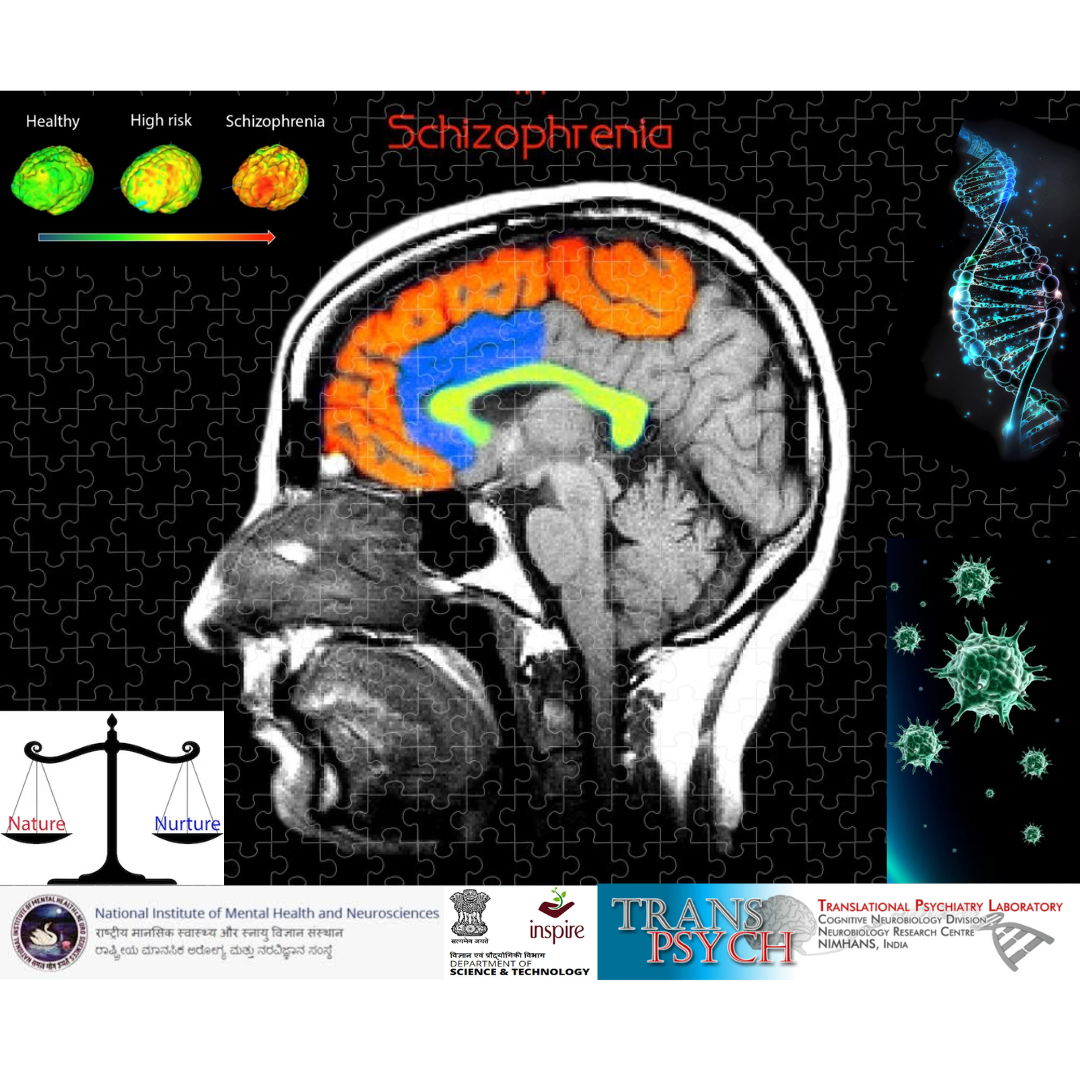
Research Fellow
National Institute of Mental Health and Neurosciences, India
Innovation in Science Pursuit for Inspired Research (INSPIRE) awarded me a 5-year research grant to pursue my graduate training. My research was focused on exploring the genetic and immunological factor underlying the onset and clinical symptoms of Schizophrenia. I primarily investigated the role of prenatal infections on neurodevelopment, its influence on the behavioural patterns by evaluating the genetic and expression profile of immune molecules of cytokines- IL-10, IL-6, TNF-α, IFN-γ and HLA-G and assessed the impact of drug interventions on the expression profile of these molecules. I also explored the role of genetic compatibility between the mother and offspring (patients) at the HLA-G gene loci in conferring risk. Parallelly I was involved in study participant recruitment,screening, assessments and sample collection. As part of screening and assessments for schizophrenia research, I worked directly with the study participants, performed phlebotomy, collected medical, treatment history and drew family pedigrees/genograms.
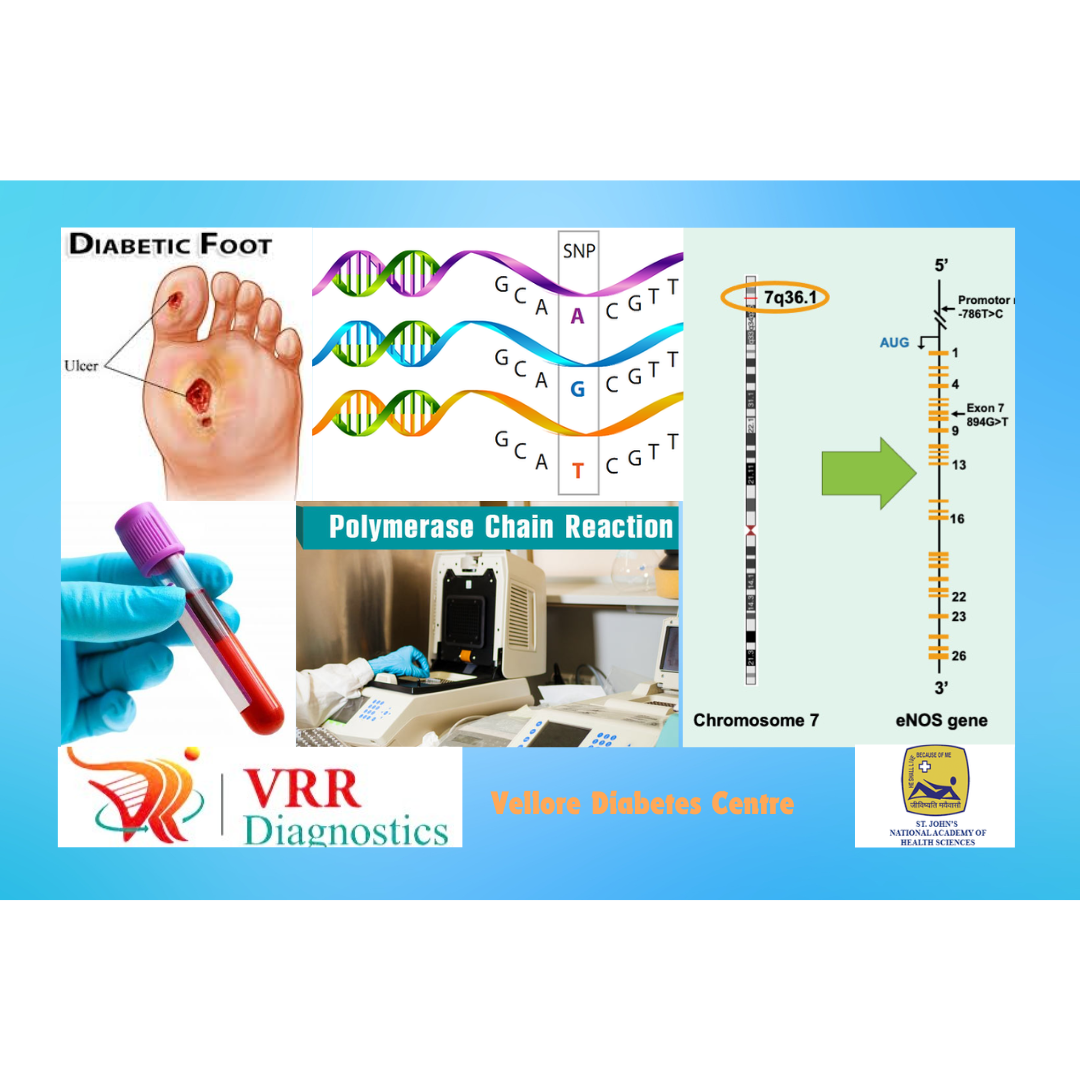
Research Trainee
VRR Diagnostics Research Laboratory, Chennai, India
In the research wing of VRR diagnostic laboratory, I worked on a project that focused on the genetic cause of Diabetic foot ulcer. I addressed the question of whether genetic polymorphisms of endothelial nitric oxide have a crucial role in the vascular endothelium to increased oxidative stress, thereby increasing the risk for diabetic foot ulcer. To facilitate this research, I independently established collaborations with St John’s National Academy of Health Sciences, Bangalore and Vellore Diabetes Centre from where I was able to obtain human specimens. During another research project at Indian Institute of Technology (IIT), Madras, I worked towards understanding the agarase or carragenase producing bacteria under halophilic conditions. The goal was to isolate these organisms to be able to exploit their function to control many applications including algae bloom, avoid biofouling etc.
Education
Ph.D in Human Genetics
Immunogenetics of Neuropsychiatry
National Institute of Mental Health & Neurosciences (NIMHANS)
M.Sc in Advanced Biochemistry
University of Madras
B.Sc in Chemistry, Zoology, Microbiology
Mount Carmel College, Bangalore University
Awards
MITACS ACCELERATE industrial postdoctoral fellowship (2021-2024)
BioTalent Canada awarded ‘BioReady Research Scientist’ paid internship progra (2018)
Awarded International Congress of Human Genetics ICHG Travel award (2016)
Awarded national travel grant from Department of Science and Technology, India, and Centre for International Cooperation in Science (CICS), India, to attend and present at ICHG Conference, Kyoto (2016)
Awarded Innovation in Science Pursuit for Inspired Research (INSPIRE) fellowship -2012-2017, Department of Science and Technology (DST), Ministry of Science and Technology, Government of India
University first rank and gold medalist in Master of Science, University of Madras (2011)
Madras University Merit Endowment Scholarship (2009-2011) for outstanding student.
Skills
Molecular biology
Molecular Biology
- Nucleic acid isolation and quantification: Automated Qiagen Prep station, Bioanalyzer, Nanodrop.
- Nanostring nCounter Assays
- Lymphocyte isolation by density gradient centrifugation method
- PCR assays (Simple & Multiplexing)
- Restriction Fragment Length Polymorphism (RFLP) assay
- Agarose gel electrophoresis
- SDS-PAGE
- Primer designing, cDNA synthesis, Quantitative Real-time PCR: TaqMan Allelic discrimination Assay & gene
expression assay - Mitochondrial DNA copy number variation assays
- Telomere length measurement assays
- In vitro transcription and translation assays
- Bacterial and mammalian cell culture (extensive)
- Transfection assays
- Protein extraction and Western blot • Patient interview and assessment scales & Dermatoglyphic assessments
(Psychiatry research) - Blood sample collection by Veni- puncture method/ Phlebotomy

Immunoassays
Immunoassays
- ELISA
- FACS
- Immunofluorescence-staining of cells
- Inverted fluorescent microscopy

Animal Studies
Animal Studies
- Qualified for use of rodents in biomedical research
- Breeding, ear clipping, IV & IP injections
Softwares
Softwares
- SPSS
- R studio
- Genetic analysis tools: PLINK, Haploview
- Image analysis using ImageJ software
- Reference managers: End note, Mendeley
- FACS analysis: CytExpert
- Gene expression analysis- Nanostring nSolver software

Mentoring
Mentoring
- Research Mentor Skool Mentor, USA
- Mentored undergraduate and high school students in carrying out in-silico research projects.
- Science instructor & Program coordinator Mad Science of greater Vancouver, BC, Canada
- Coordinated Mad Science programs in lower mainland
- Delivered hands-on science experiments to spark imaginative learning

Research Administration
Research Administration
As Laboratory manager for 4 years , have handled the following duties independently:
- Led meetings with stakeholders and collaborators
- Supervised research personnel and students- Postgrad, undergrad and short term students
- Incharge of Laboratory inventory management and budgeting
- Responsible for laboratory compliance and bioethics
- Oversaw management of laboratory equipments and troubleshooting
- Managed mice breeding colonies and routinely genotyped to ensure the proper maintenance of inbred strains
Scientific Contributions
Tomlinson C, Rajasekaran A, Khatiz A, Farmilo A.J, Gris P, Matthews A, Amrani A, & Gris D
A Convenient Analytic Method for Gel Quantification Using ImageJ Paired with Python or R. PLOS ONE (2024)
Khan F U, Khongorzul P, Raki A A, Rajasekaran A, Amrani A, Gris D
Khan F U, Khongorzul P, Raki A A, Rajasekaran A, Amrani A, Gris D
Samra S K, Rajasekaran A, Sandford A J, Ellis A K, Tebbutt, S. J.
Rajasekaran A, Shivakumar V, Kalmady S V, Parlikar R, Chhabra H, Prabhu A et al
Shivakumar V, Rajasekaran A, Subbanna M, Kalmady S V, Venugopal D, Agrawal R et al.
Rajasekaran A, He D, Yue A, Singh A, Shannon C. P, FitzGerald J. M, Boulet L-P, O’Byrne P. M, Gauvreau G. M, Tebbutt, S. J
Shivakumar V, Debnath M, Venugopal D, Rajasekaran A, Kalmady S V, Subbanna M, et al
Shivakumar V, Kalmady S V, Rajasekaran A, Chhabra H, Amaresha A C, Narayanaswamy J C, et al.
Kalmady S V, Agarwal R, Venugopal D, Shivakumar V, Amaresha A C, Agarwal S M, Subbanna M, Rajasekaran A, et al.
Rajasekaran A, Shivakumar V, Kalmady S V, Narayanaswamy J C, Subbana M, Venugopal D, et al.
Rajasekaran A, Shivakumar V, Kalmady S V, Narayanaswamy J C, Subbana M, Venugopal D, et al
Rajasekaran A, Shivakumar V, Kalmady S V, Narayanaswamy J C, Venugopal D, Amaresha A C, et al
Rajasekaran A, Venkatasubramanian G, Berk M, Debnath M.
Chhabra H, Shivakumar V, Agarwal S M, Bose A, Venugopal D, Rajasekaran A, et al.
Presentations
mRNA Conference 2023 (28 – 29 August 2023)
Cassidy Tomlinson, Alfred James Farmilo, Marjo Piltonen, Amanda Matthews, Diego
Sobera, Anastasia Mickiewicz, Jewel Mickiewicz, Ashwini Rajasekaran, Pavel Gris &
Denis Gris. “Deuterium Protium Exchange Protects mRNA From Thermal & Enzymatic Hydrolysis And Improves Translational Efficiency Of mRNA Molecules.”
Canadian Chemistry Conference and Exhibition 2025 (June 15-19), Ottawa [Accepted]
Yulia A. Vlasenko, Ashwini Rajasekaran, Denis Gris,Pavel Gris, Graham K. Murphy.“Study of the nature of mRNA stabilization in heavy water by hydrogen deuterium exchange mass spectrometry.”
EnergEn 2023, 18–20 October 2023, Băile Govora, Romania
Pavel Gris, Alfred James Farmilo, Cassidy Tomlinson, Marjo Piltonen, Amanda Matthews, Diego Sobera, Anastasia Mickiewicz, Jewel Mickiewicz, Ashwini
Rajasekaran, Denis Gris. “Effect Of Deuterium Protium Exchange On mRNA Translational Efficiency.” at XXIV
the International Conference “New Cryogenic and Isotope
Technologies for Energy and Environment”
EnergEn 2023, 18–20 October 2023, Băile Govora, Romania
Pavel Gris, Alfred James Farmilo, Cassidy Tomlinson, Marjo Piltonen, Amanda Matthews, Diego Sobera, Anastasia Mickiewicz, Jewel Mickiewicz, Ashwini
Rajasekaran, Denis Gris. “Deuterium Protium Exchange Protects mRNA From Thermal & Enzymatic Hydrolysis And Improves Translational Efficiency Of mRNA Molecules.” at
XXIV the International Conference “New Cryogenic and Isotope Technologies for Energy
and Environment”